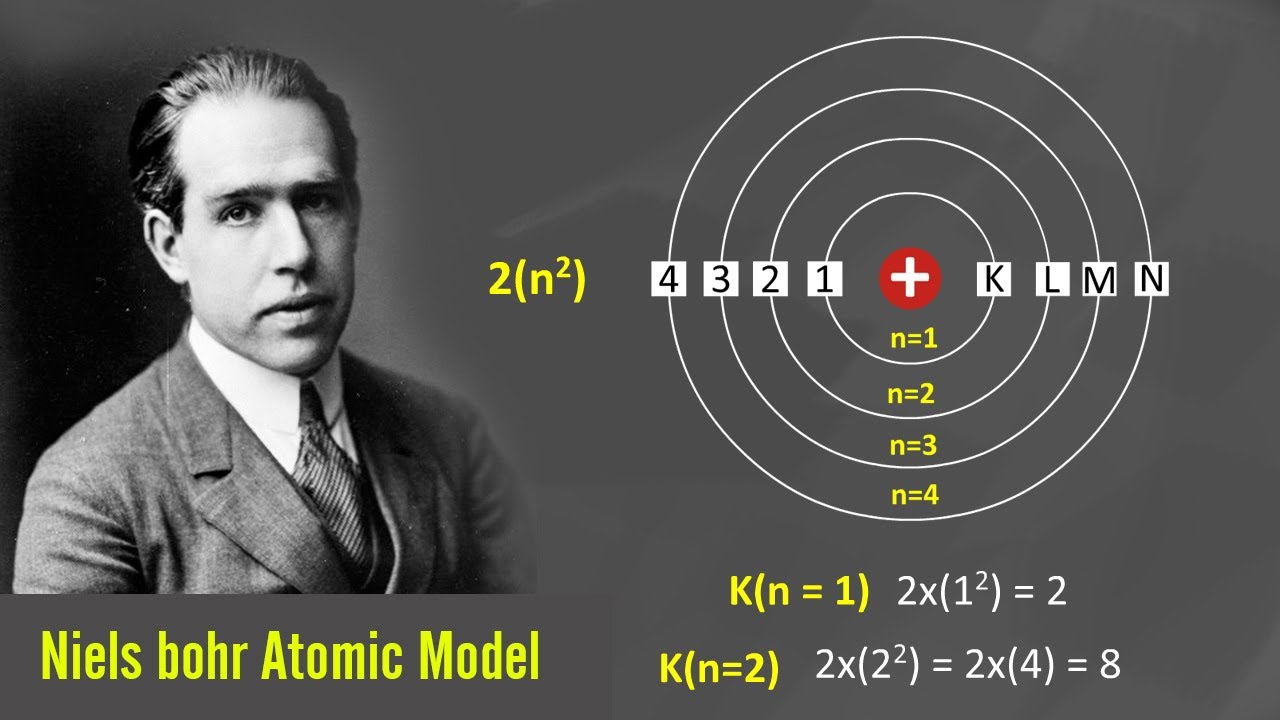
What Is Bohr Atomic Model Bohr s model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells or orbits around the nucleus Bohr s model calculated the following energies for an electron in the shell n E n 1 n 2 13 6 eV
The Bohr model Google Classroom Learn how Bohr models are used to represent atoms Atoms are way too small to see with the naked eye and even most microscopes So we represent atoms using models Models help us visualize atomic structure They also help us explain and predict the behavior of atoms Sep 20 2022 0183 32 Bohr s Atomic Model Following the discoveries of hydrogen emission spectra and the photoelectric effect the Danish physicist Niels Bohr 1885 1962 proposed a new model of the atom in 1915 Bohr proposed that electrons do not radiate energy as they orbit the nucleus but exist in states of constant energy that he called stationary states
What Is Bohr Atomic Model
 What Is Bohr Atomic Model
What Is Bohr Atomic Model
https://thechemistrynotes.com/wp-content/uploads/2023/05/image-68-1024x824.png
Jun 20 2015 0183 32 Bohr s proposal explained the hydrogen atom spectrum the origin of the Rydberg formula and the value of the Rydberg constant Specifically it demonstrated that the integers in the Rydberg formula are a manifestation of quantization
Pre-crafted templates provide a time-saving option for producing a diverse series of documents and files. These pre-designed formats and designs can be made use of for various personal and professional jobs, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, simplifying the content production procedure.
What Is Bohr Atomic Model

M8 S7 Bohr s And Rutherford s Atomic Models And Their Limitations

Bohr Model Of The Atom
Bohr s Model Of An Atom Bohr Model Class 9 Atom Tutorial YouTube

Bohr s Atomic Model Overview Importance Expii

Bohr s Atomic Model Postulates Diagram Limitations
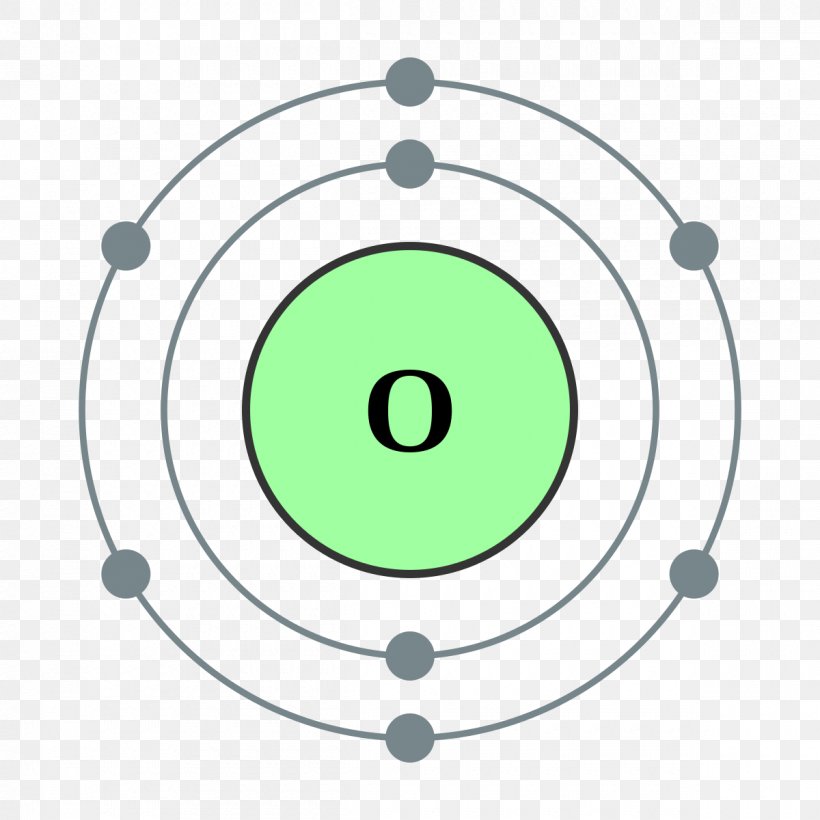
Bohr Model Chemical Element Oxygen Atomic Theory PNG 1200x1200px

https://en.wikipedia.org/wiki/Bohr_model
In atomic physics the Bohr model or Rutherford Bohr model of the atom presented by Niels Bohr and Ernest Rutherford in 1913 consists of a small dense nucleus surrounded by orbiting electrons It is analogous to the structure of the Solar System but with attraction provided by electrostatic force rather than gravity and with the

https://byjus.com/chemistry/bohrs-model
Bohr s Model of an Atom Bohr s model consists of a small nucleus positively charged surrounded by negative electrons moving around the nucleus in orbits Bohr found that an electron located away from the nucleus has more energy and the electron which is closer to nucleus has less energy

https://www.thoughtco.com/bohr-model-of-the-atom-603815
Jan 27 2020 0183 32 The Bohr Model has an atom consisting of a small positively charged nucleus orbited by negatively charged electrons Here s a closer look at the Bohr Model which is sometimes called the Rutherford Bohr Model Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915
https://blog.prepscholar.com/bohr-model
The Bohr Model is known as a planetary model because these orbits look similar to that of planets orbiting the sun The Bohr Model Explained There are three main factors that characterize the Bohr model First in the Bohr Model the

https://www.toppr.com//bohrs-model-of-atom
What is Bohr s Model of an Atom According to the Bohr Atomic model a small positively charged nucleus is surrounded by revolving negatively charged electrons in fixed orbits He concluded that electron will have more energy if it is located away from the nucleus whereas electrons will have less energy if it located near the nucleus
The Bohr Model is a structural model of an atom The model was proposed by physicist Niels Bohr in 1913 In this model the electrons travel around the nucleus of an atom in distinct circular orbits or shells The model is also The Bohr model named after Danish physicist Niels Bohr of an atom has a small positively charged central nucleus and electrons orbiting in at specific fixed distances from the nucleus
Jul 28 2016 0183 32 Proposed by Danish physicist Niels Bohr in 1913 this model depicts the atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits defined by their