Chemical Bonding Exercises With Answers 1 Explain how and bonds are similar and how they are different 2 Draw a curve that describes the energy of a system with H and Cl atoms at varying distances Then find the minimum energy of this curve two ways a Use the bond energy found in Table 8 1 to calculate the energy for one single HCl bond Hint How many bonds are in a mole
Answer Use the knowledge of coordinate covalent bonds and explain the formation of the following polyatomic ions a Formation of NH 4 by the reaction between NH3 and water H b Formation of H3O by the reaction between H2O and H c Formation of BF 4 by the reaction between BF3 and F Answer Course AP 174 College Chemistry gt Unit 2 Lesson 1 Types of chemical bonds Ionic bonds Covalent bonds Metallic bonds Predicting bond type metals vs nonmetals Predicting bond type electronegativity Types of chemical bonds Science gt
Chemical Bonding Exercises With Answers
 Chemical Bonding Exercises With Answers
Chemical Bonding Exercises With Answers
https://i.pinimg.com/originals/e3/7c/5b/e37c5b35b3eea20c4c9a7e53fa4e632c.jpg
Why Q 8 State the difference between bonding and antibonding molecular orbitals Q 9 Why cannot a single bond be a pi bond Q 10 Why is glycerol more viscous than ethanol Q 11 Describe why p hydroxybenzaldehyde is a high melting solid and o hydroxybenzaldehyde is a liquid at room temperature
Pre-crafted templates offer a time-saving option for developing a diverse variety of files and files. These pre-designed formats and layouts can be utilized for numerous personal and professional projects, consisting of resumes, invites, flyers, newsletters, reports, presentations, and more, streamlining the material development procedure.
Chemical Bonding Exercises With Answers

Ionic Bonding Worksheets Answer Key
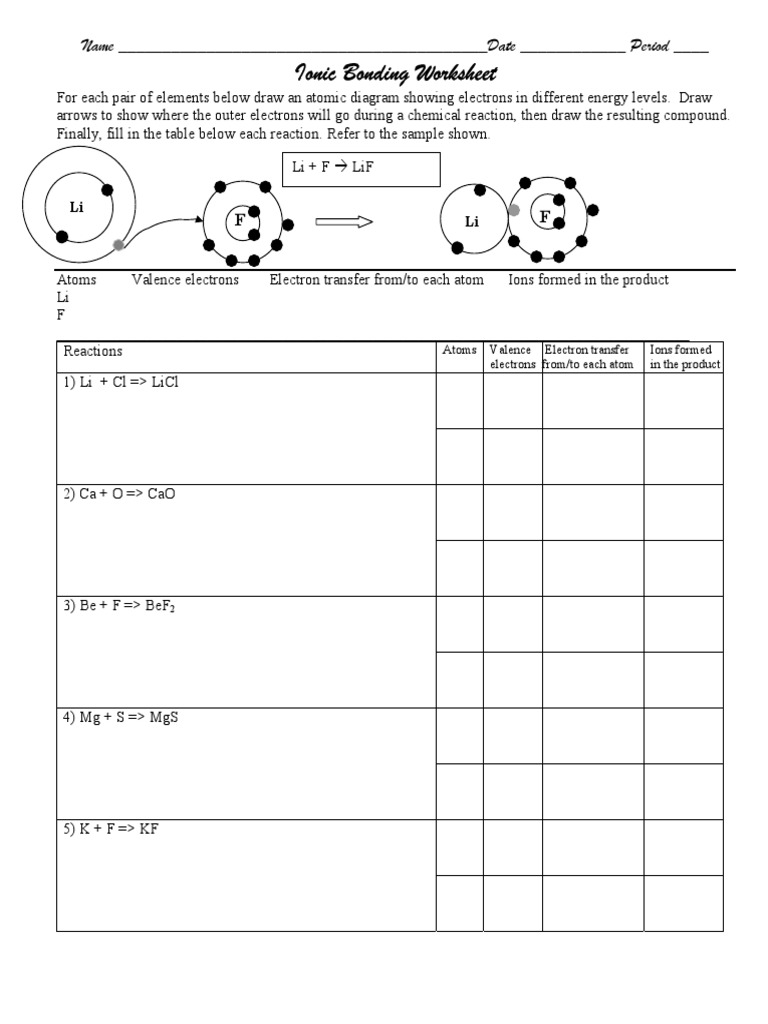
Ionic Bonding Worksheet 1
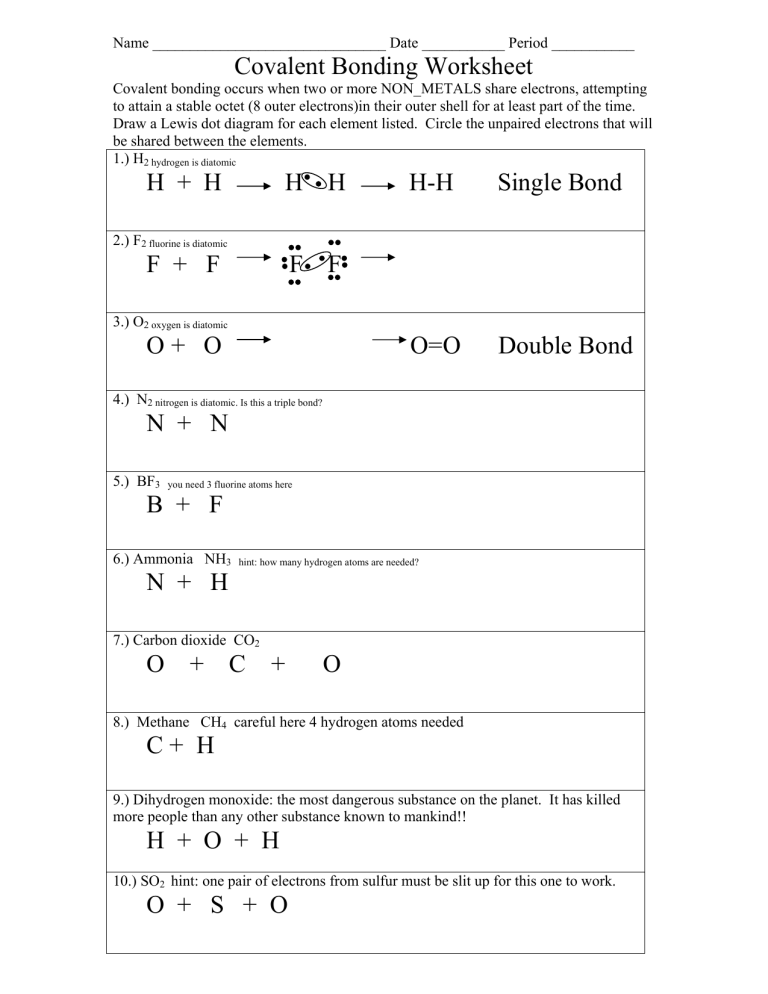
Covalent Bonding Worksheet
Types Of Covalent Bonds Worksheet

Covalent Bonding Worksheet And Answers A Guide To Understanding

Naming Covalent Compounds Worksheet Answers Ionic And Covalent Bonds

https://chem.libretexts.org/Bookshelves/General
Jun 14 2022 0183 32 The Lewis electron system is a simplified approach for understanding bonding in covalent and ionic compounds Why do chemists still find it useful Is a Lewis dot symbol an exact representation of the valence electrons in

https://www.khanacademy.org//e/chemical-bonds
Choose 1 answer A hydrogen atom with a slight positive charge is attracted to a negative charge of another molecule or atom A A hydrogen atom with a slight positive charge is attracted to a negative charge of another molecule or atom Two atoms share electrons so they can fill their outer shells B
https://www.learncbse.in/ncert-solutions-for-class
Question 1 Explain the formation of a chemical bond Answer According to Kossel and Lewis atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration

https://chem.libretexts.org/Bookshelves
4 days ago 0183 32 Exercises Electron Transfer Ionic Bonds Answers Exercises Covalent Bonds Answers Exercises Other Aspects of Covalent Bonds Answers Exercises Violations of the Octet Rule Answers Exercises Molecular Shapes Answers Additional Exercises Answers

https://byjus.com/ncert-solutions-class-11
The NCERT Solutions for Class 11 Chemistry Chapter 4 provided on this page feature the following types of questions Drawing Lewis dot symbols for atoms molecules and polyatomic ions Questions on bond parameters Expressing resonance with the help of Lewis structures Questions related to dipole moment bond polarity and polar covalent
4 days ago 0183 32 The first description of covalent bonding was provided by Lewis in terms of the sharing of electron pairs between atoms and he related the process to the attainment of noble gas configurations by reacting atoms as a result of sharing of electrons Jan 6 2014 0183 32 Chemical bonding exercise with solutions 1 Class XI Chapter 4 Chemical Bonding and Molecular Structure Chemistry Question 4 1 Explain the formation of a chemical bond Answer A chemical bond is defined as an attractive force that holds the constituents atoms ions etc together in a chemical species
Oct 2 2020 0183 32 Chemical Bonding Unit Plan 127 Favorites LESSON PLAN in Intermolecular Forces Polarity Naming Compounds Molecular Formula Covalent Bonding Ionic Bonding Molecular Structure VSEPR Theory Molecular Geometry Resonance Electronegativity Metallic Bonding Unit Plans Last updated October 02