Difference Between Intensive And Extensive Properties Dec 15 2017 0183 32 Physical properties are in two types as intensive properties and extensive properties The main difference between intensive and extensive properties is that intensive properties do not depend on the amount of matter whereas extensive properties depend on the amount of matter
Intensive and extensive properties provide valuable insights into the nature and behavior of matter Intensive properties are independent of the sample size while extensive properties depend on the quantity of the substance being considered Aug 12 2014 0183 32 Key Difference Intensive properties refer to properties that are independent compared to the size or quantity of the substance Extensive properties refer to properties that are dependent on the size or quantity of the substance
Difference Between Intensive And Extensive Properties
:max_bytes(150000):strip_icc()/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png) Difference Between Intensive And Extensive Properties
Difference Between Intensive And Extensive Properties
https://www.thoughtco.com/thmb/baWwJ1LXMNR6oKRo-pFy7rylCic=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png
Jun 11 2023 0183 32 The difference between extensive and intensive properties is that extensive properties depend on the amount of matter but intensive properties do not Also the size of extensive properties changes but the size of intensive properties is fixed and does not change
Pre-crafted templates offer a time-saving option for developing a varied range of documents and files. These pre-designed formats and layouts can be utilized for numerous personal and professional jobs, including resumes, invites, leaflets, newsletters, reports, presentations, and more, improving the content production process.
Difference Between Intensive And Extensive Properties
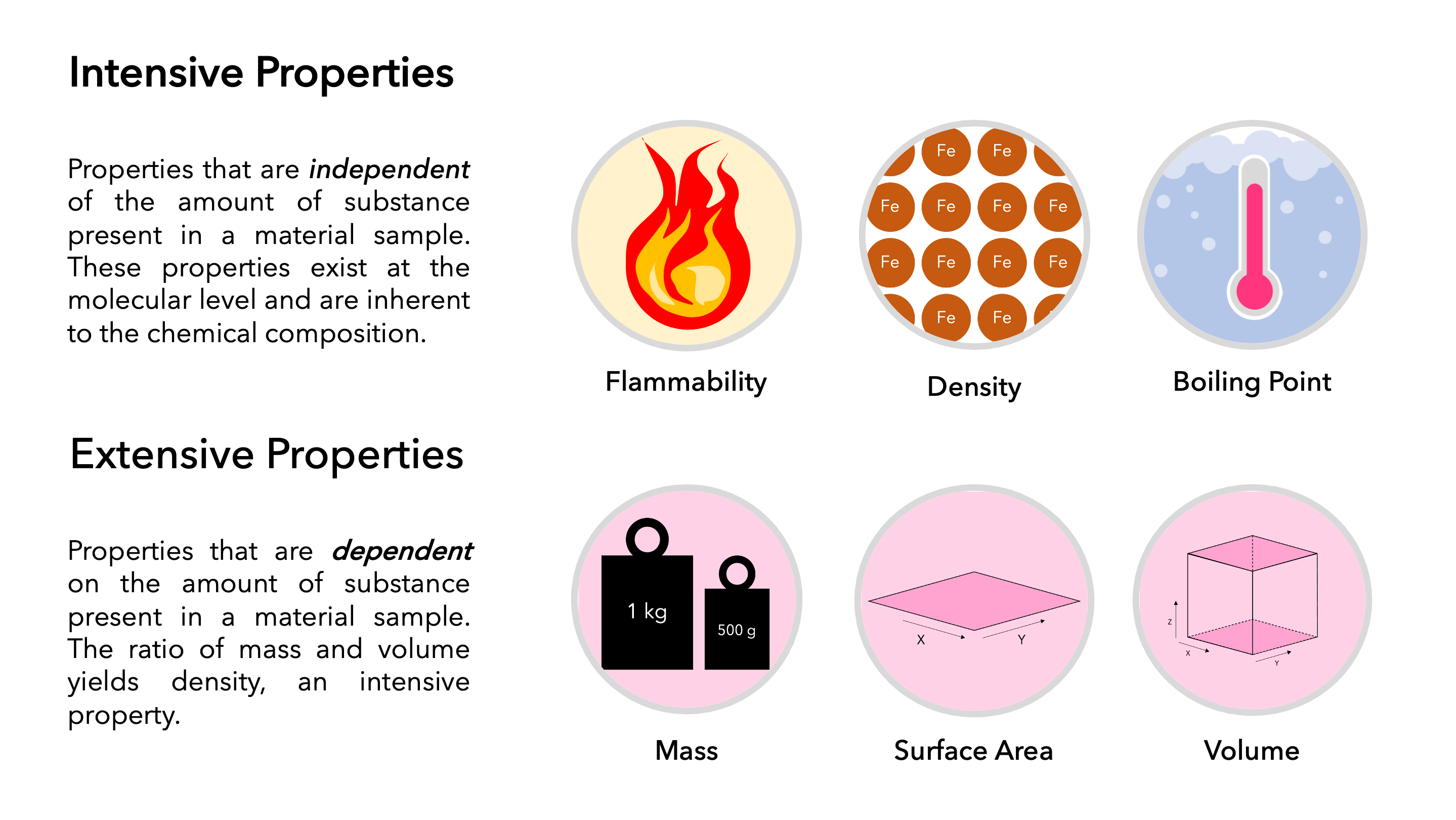
Describe The Difference Between An Extensive Property And Intensive

Difference Between Intensive And Extensive Properties FDB

Is The Weight Of A System An Extensive Or Intensive Property
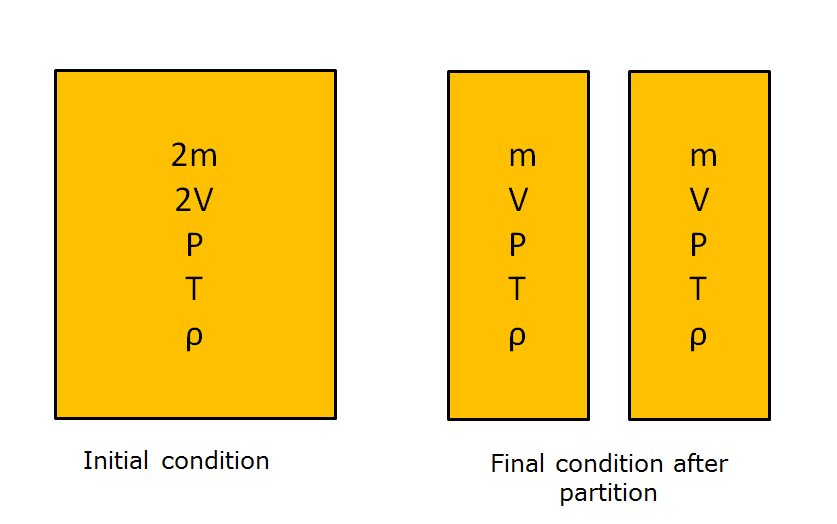
Difference Between Intensive And Extensive Properties Mechanical Booster

PPT PHYSICAL PROPERTIES Glass And Soil PowerPoint Presentation ID

Difference Between Extensive And Intensive Properties Hindi YouTube
:max_bytes(150000):strip_icc()/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png?w=186)
https://www.thoughtco.com
Jun 1 2024 0183 32 The two types of physical properties of matter are intensive properties and extensive properties Intensive properties do not depend on the quantity of matter Examples include density state of matter and temperature Extensive properties do depend on sample size Examples include volume mass and size

https://chem.libretexts.org › Bookshelves
An extensive property is a property that depends on the amount of matter in a sample Mass and volume are examples of extensive properties An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount

https://sciencenotes.org › intensive-extensive-properties
Mar 18 2020 0183 32 Extensive and intensive properties are the two types of physical properties of matter Intensive properties do not depend on the amount of matter in a substance Examples include state of matter temperature and density

https://en.wikipedia.org › wiki › Intensive_and_extensive_properties
The distinction between intensive and extensive properties has some theoretical uses For example in thermodynamics the state of a simple compressible system is completely specified by two independent intensive properties along with one extensive property such as mass

https://www.geeksforgeeks.org › intensive-and
Jan 15 2024 0183 32 Intensive property is a property of matter that does not change with the size of the sample For example pressure density etc Extensive property is a property of matter that depends upon the amount of substance i e varies with the size of the material like weight volume mass etc
What is the difference between intensive property and extensive property An extensive property is a property that depends on the amount of matter in a sample Mass and volume are examples of extensive properties Intensive Properties The properties which are independent of the mass of the system Temperature pressure and density are the intensive properties Extensive Properties The properties which depend on the size or extent of the system are called extensive properties For example total mass total volume and total momentum Also Read
Feb 13 2024 0183 32 The basic difference between the extensive type property and intensive type property is that the extensive property varies or depends on the quantity or amount of substance while the intensive property never or does not varies or depend on the quantity of substance