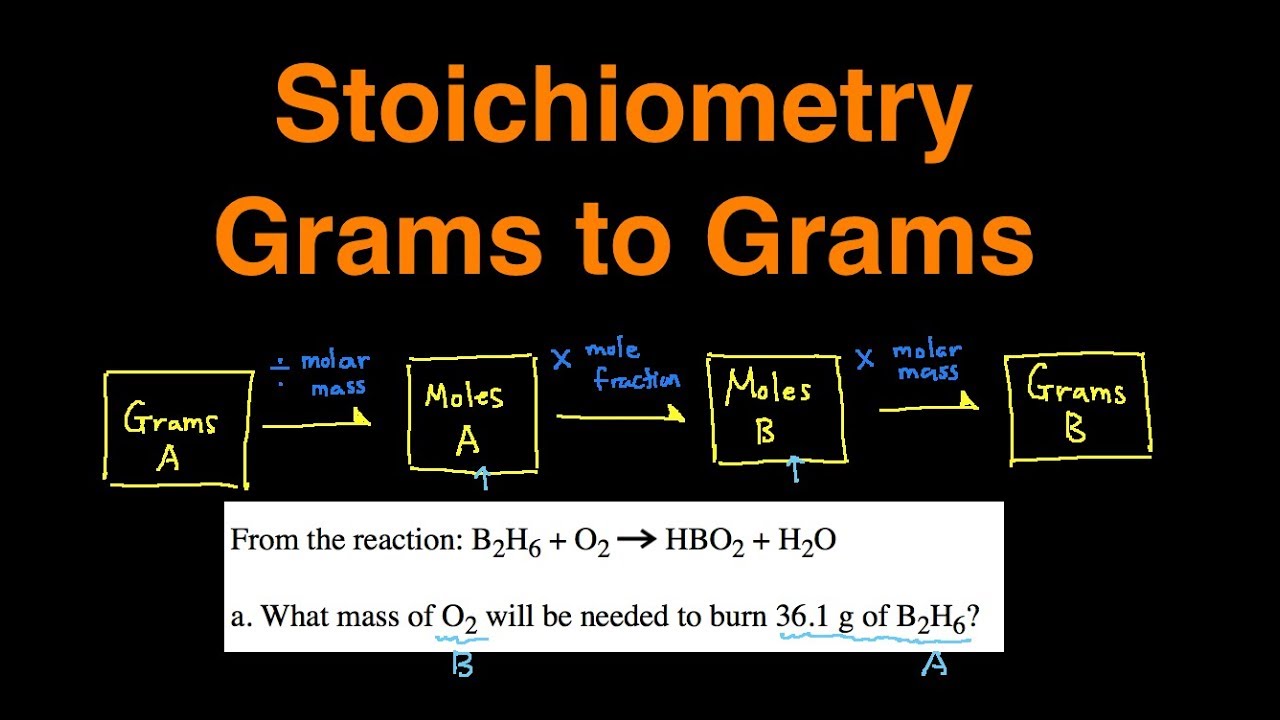
How To Do Stoichiometry Grams To Grams Step 1 Convert known reactant mass to moles In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio we first need to know the quantity of H A 2 SO A 4 in moles We can convert the 3 10 grams of H A 2 SO A 4 to moles using the molar mass of H A 2 SO A 4 98 08 g mol
Mass to mass conversion from a chemical equation A chemical equation gives a mole to mole conversion factor If the given substance is in grams and the desired substance is also in grams then two additional conversion factors based on the molar masses are needed That is the following conversions are needed Using Balanced Chemical Equations grams to grams This page provides exercises in using chemical reactions to relate the masses of two substances When you press quot New Problem quot a balanced chemical equation with a question will be displayed Determine the correct value of the answer enter it in the cell and press quot Check Answer quot
How To Do Stoichiometry Grams To Grams
 How To Do Stoichiometry Grams To Grams
How To Do Stoichiometry Grams To Grams
https://i.ytimg.com/vi/Bj3fr5pEn7U/maxresdefault.jpg
A B C D The concept of stoichiometry will help us answer questions like if x grams of A is present then how many grams of C or D or both will be produced if y grams of B is present then how many grams of C or D or both will be produced
Pre-crafted templates offer a time-saving option for producing a diverse range of documents and files. These pre-designed formats and designs can be used for different personal and professional projects, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, simplifying the content development procedure.
How To Do Stoichiometry Grams To Grams

Stoichiometry Grams To Moles Moles To Grams Or Moles To Moles 1 Or

Stoichiometry Problems Grams To Liters Of A Gas YouTube

Stoichiometry Mole To Mole Grams To Grams Mole Ratio Practice
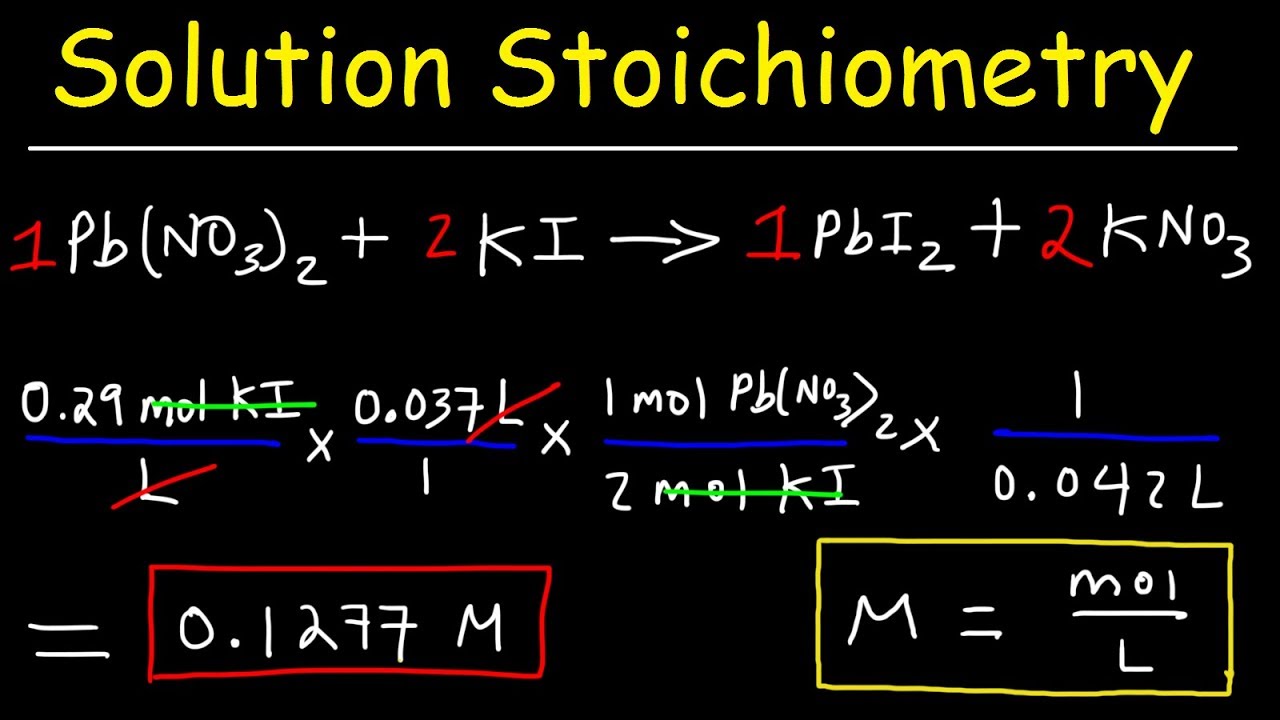
Solution Stoichiometry Finding Molarity Mass Volume YouTube

Chemical Reactions 7 Of 11 Stoichiometry Grams To Moles YouTube

Moles To Grams Stoichiometry YouTube

https://www.youtube.com/watch?v=OkWV9d7Z6VA
Mar 28 2018 0183 32 Stoichiometry Converting Grams to Grams How many grams of Ca OH 2 are needed to react with 41 2 g of H3PO4 The equation is 2 H3PO4 3 Ca OH 2 Ca3 PO4 2 6 H2O more How many
https://www.wikihow.com/Do-Stoichiometry
Dec 2 2022 0183 32 Convert grams of a substance to moles using molar mass Using the molar mass as a conversion factor you can calculate the number of moles present in the stated number of grams of the species Divide the known

https://chem.libretexts.org/Bookshelves/General
Steps to getting this answer Since you cannot calculate from grams of reactant to grams of products you must convert from grams of C 3H 8 to moles of C 3H 8 then from moles of C 3H 8 to moles of H 2O Then convert from moles of H 2O to grams of H 2O Step 1 200 g C 3H 8 is equal to 4 54 mol C 3H 8

https://chem.libretexts.org/Courses/Bellarmine
Flowchart of steps in stoichiometric calculations Step 1 grams of A is converted to moles by multiplying by the inverse of the molar mass Step 2 moles of A is converted to moles of B by multiplying by the molar ratio Step 3 moles of

https://www.youtube.com/watch?v=dAaputfjDKU
Aug 4 2017 0183 32 How to Convert Grams to Grams Stoichiometry Examples Practice Problems Questions Explained Conquer Chemistry 26 5K subscribers Subscribe Subscribed 382 34K views 6 years ago
Stoichiometry Grams to Grams using a balanced equation Video 1 Chemistry Whitwell High School UTC University of Tennessee at Chattanooga www whitwell What is used as the conversion factor for each Answers g mol mol g g mol molar mass mol mol mol ratio from coefficients mol g molar mass Practice Complete the following questions using the information you learned during the lesson activity Questions 2 Ga2O 4 Ga 3 O 3 2
To convert between moles and grams multiply moles by the molar mass to get grams or divide grams by the molar mass to get moles For example lets say we have 100g of MgCl2 and want to convert it to the number of moles 100 95 211 1 05 moles